It seems that you can’t go anyplace these days without hearing about medications like Ozempic and Mounjaro. It naturally raises the question of what they are and how their high prices are justified.
While these synthetic peptides are innovative and have many regulations for their compliance, they represent only the tip of the iceberg. To properly understand this new Era of medicine, we must examine an even more difficult and pricey class of treatments: biologics.
So let me take you for a closer look at what biologics are, why they expanded the market, and the very real challenges that make them both remarkable and extremely tough to manufacture. Understanding this explains not only their expenses, but also their great value in saving millions of people.
What are biologics?
A biologic is a type of medicine or vaccine that originates from living sources. These products can be developed from proteins, sugars, DNA, cells, or even whole tissues. The source material may come from humans, animals, or microorganisms such as bacteria and viruses. They are no longer the future; they are the present. With the market expected to rise from USD 487 billion in 2025 to over USD 1.14 trillion by 2034, innovation in biologics is reshaping global healthcare.
The Manufacturing Journey of a Biologic Drug
This is not a typical journey because, as you know, it is for human use, and you are working with proteins and cells to express your target biologic, so every step is subject to strict regulations. You also need to be aware that every biologic drug is exposed during this journey into the “valley of death,” where it begins as a successful drug in the lab with cells, but when we want to scale it up in large bioreactors, it is very costly, time-consuming, and exhausting compared to any other chemical drug.
And to summarize this journey, there are three main phases :
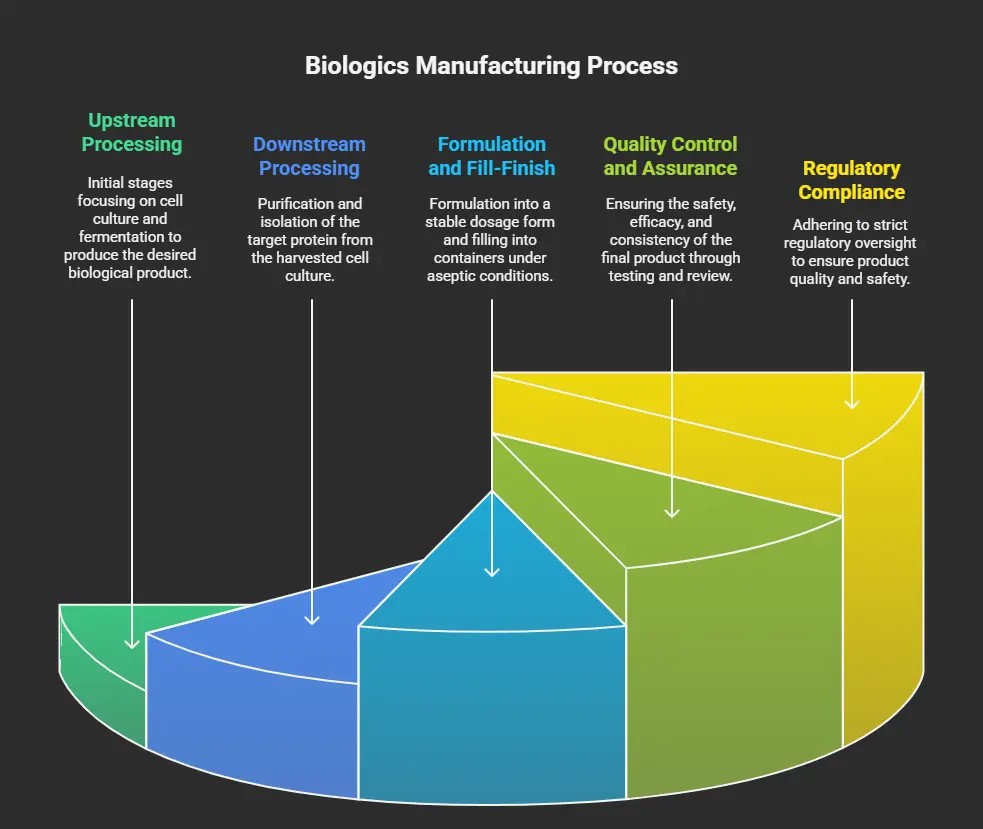
Phase 1: Upstream Processing (Creation)
This phase focuses on generating the therapeutic protein using living systems:
- Cell Line Development: Genetic engineering creates a high-performing host cell (often CHO) containing the gene for the desired protein. This champion cell forms the Master Cell Bank.
- Scale-Up: Cells are grown gradually in nutrient medium, moving from small flasks to massive, highly controlled bioreactors.
- Bioreaction: Cells multiply and actively secrete the target biologic protein into the culture medium over several days or weeks.
Phase 2: Downstream Processing (Purification)
This is the painstaking and expensive phase of separating and refining the protein from the cell broth:
- Harvesting: To produce a clear liquid harvest, filters and centrifuges are used to eliminate the cells and big debris.
- Purification (Chromatography): To achieve over 99.9% purity, the harvest is run through a series of specialized columns (resins) that selectively bind and separate the target biologic from contaminants.
- Viral Inactivation & Removal: To neutralize and eliminate any possible viruses, critical safety procedures include chemical treatment and extremely stringent nano-filtration.
Phase 3: Final Steps (Formulation and Finish)
The pure protein is prepared for safe and effective patient use:
- Formulation: The biologic is combined with stabilizing excipients to ensure stability, control PH, and reach the exact therapeutic concentration.
- Filling & Packaging: The drug is sterile-filtered and aseptically filled into vials or syringes, followed by rigorous visual inspection.
- Cold Chain: The final product is immediately placed into an unbroken cold storage chain 2-8 C to maintain the protein’s fragile structure until delivery
Sounds easy when you read it but what is the challenges in real life ?
But actually It’s messy, sensitive, and every tiny change can flip the whole process upside down.
Scale Up Challenges
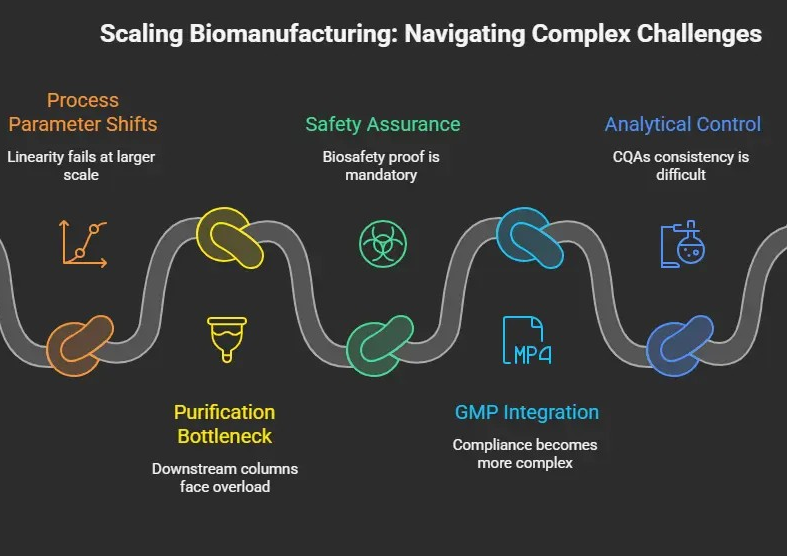
Here’s what happens in real life:
- At the Lab scale, cells are angels in a 2L flask, but when it is 2000L or 10000L reactors, all the game changes as the growth rate, oxygen, and shearing stress affect all the productivity and even the protein folding.
- Process Parameters Don’t Transfer Linearly.
Whatever worked in the lab won’t behave the same on the plant floor; the struggle will be with the :
- oxygen gradients
- CO₂ accumulation
- slower mass transfer
- longer mixing times
This directly affects CQAs (Critical Quality Attributes).
- A bottleneck is now your purification
Your downstream columns suddenly need to handle massive protein loads.
And the Challenges will be:
- resin capacity limits
- pressure drops
- long cycle times
- viral filtration clogging
- maintaining batch consistency
4. Safety Must Be Proven at Scale
Every scale change requires showing:
- equipment + environment meet biosafety requirements
- Proving zero contamination of the virus and other impurities
5. GMP Integration Gets Way More Complex
Bigger scale means bigger compliance headaches:
- Environmental monitoring
- Equipment qualification
- Raw material variability
- Data integrity
- Operator training
6. Analytical and Quality Control Challenges
- Ensuring consistent Critical Quality Attributes (CQAs) across batches is complex.
- Methods must detect small variations in purity, potency, aggregation, glycosylation, or host-cell impurities.
- Large-scale production generates high volumes of data, requiring strict GMP-compliant data management.
Summary
Scaling up a biologic is more equivalent to attempting to expand a delicate living thing from a teacup into an ocean and expecting it to behave precisely the same. It goes beyond simply “making more of the same product.” Cell growth, purification, formulation, and quality control all become crucial balancing acts at every stage of the process.
A small change in temperature, a slightly slower mixing pattern, or a modest adjustment in pH can throw the entire process off course, impacting safety, effectiveness, and eventually regulatory approval. Because they need accuracy, ongoing observation, and a thorough comprehension of living systems, biologics are at the summit of the complexity pyramid.
But let me ask a question after reading this topic, and the stability studies topic mentioned below
Stability Studies of Pharmaceuticals: Ensuring Efficacy and Safety Over Time
I’d be interested to hear your views on how stability studies fit into the biologics era.
References